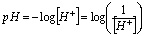pH Adjustment
Maintaining the correct pH level is essential for efficient water treatment, whether it’s for potable water or wastewater. The pH of water directly influences the effectiveness of treatment processes and its eventual discharge into the environment. pH adjustment is a critical part of wastewater treatment, helping to neutralize harmful substances and protect both equipment and the environment.
Because pH is simply another means of referring to the hydrogen ion concentration, acidic and basic solutions can be distinguished on the basis of pH values:
| pH < 7 pH = 7 pH > 7 | Acidic solution Neutral solution Basic solution |
What Is pH Adjustment in Wastewater Treatment?
In wastewater treatment, pH refers to the measure of acidity or basicity of the water. If the pH is too high (basic) or too low (acidic), it can cause significant issues in the treatment process, including the malfunction of treatment equipment and an inability to meet environmental regulations. pH adjustment in wastewater treatment is crucial for making sure that treated water meets safety standards before being released or reused.
pH levels in wastewater can vary greatly depending on the source of the water, such as industrial processes, agriculture runoff, or domestic waste. To achieve the desired pH level, substances such as acids or bases are added to either lower or raise the pH. Effective pH management plays a major role in protecting natural water bodies and preserving ecosystems.
Methods for pH Adjustment in Water Treatment
There are several methods used for pH adjustment in water treatment. The most common approaches include:
- Acidic pH Adjustment: When the water is too basic, acids such as sulfuric acid or hydrochloric acid are introduced to lower the pH. This method is often used in industries where alkaline chemicals are prevalent.
- Alkaline pH Adjustment: If the water is too acidic, bases substances like sodium hydroxide (caustic soda) or lime (calcium oxide) are added to raise the pH to a neutral or optimal level. This is particularly important in areas dealing with wastewater from industrial activities.
- Automatic pH Control Systems: These systems continuously monitor pH levels and automatically adjust them by adding acids or bases as needed. This helps maintain a consistent pH range and makes sure the treatment process is efficient and cost-effective.
- Manual pH Control: For smaller systems or less complex wastewater treatment needs, manual methods may be used, where operators add chemicals based on regular pH measurements.
Each of these methods plays an important role in maintaining water quality and treatment effectiveness, depending on the specific requirements of the water being treated.
The Importance of pH Adjustment in Wastewater Treatment
pH adjustment is essential for several reasons. First, it helps meet environmental standards, preventing pollution and protecting aquatic life. Wastewater that has too high or low a pH can harm ecosystems when released into rivers or lakes, as it can affect the solubility of metals and the toxicity of certain chemicals.
Secondly, pH adjustment in wastewater treatment prevents damage to treatment equipment. Extremes in pH can corrode pipes, tanks, and filtration systems, leading to costly repairs and increased maintenance efforts. By keeping the pH within an optimal range, the lifespan of treatment equipment is extended, and operational costs are reduced.
Lastly, pH adjustment in wastewater can improve the overall efficiency of treatment processes. Certain treatment methods, such as coagulation and flocculation, work better within specific pH ranges. Without proper pH control, these processes may not function effectively, leading to inadequate treatment.
Make pH Adjustment Effortless with Complete Water Services, LLC
If you need pH adjustment wastewater solutions for your wastewater or water treatment systems, reach out to Complete Water Services. Our experts are ready to assist you with tailored solutions that fit your unique needs. Contact us today for more information or to schedule a consultation.