Coagulation & Flocculation
The coagulation and flocculation processes chemically convert colloidal solids into larger solids for separation by settling or flotation. Colloidal solids are very small, finely dispersed solids that remain in suspension for long periods of time due to their size and electrical charge. Coagulation is the destabilization of colloidal solids so that particle growth can occur; clumping together very fine particles into larger particles (pin floc). Flocculation is the process by which the colloidal solids agglomerate (clump together) into larger solids that can be more readily removed. The pin floc clump together into larger particles for removal by dissolved air flotation (DAF) or gravity separation.
Coagulation is accomplished by rapidly mixing a coagulant with the wastewater after pH adjustment. Commonly used coagulants include ferric chloride (FeCl3), ferric sulfate (FeSO4) or aluminum sulfate Al2(SO4)3.
Flocculation is accomplished by slowly mixing a flocculent into the waste stream after the coagulant is added. Flocculants are long-chain polyelectrolyte (polymers) that are synthetically manufactured for solids removal in water and wastewater. Both coagulation and flocculation are very pH dependent. Coagulation and flocculation will not occur if the pH is outside of established ranges.
System Description
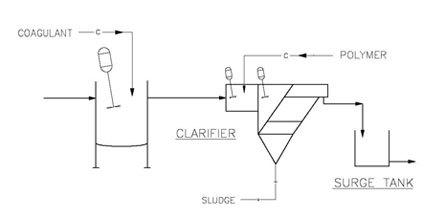
The figure below shows a simple coagulation and flocculation system integral to an inclined plate clarifier. These chemicals may also be injected into a pipe flocculator- type dosing system found on many DAF systems.